Incandescence & Luminescence
Light is a form of energy. To create light, another form of energy must be supplied. There are two common ways for this to occur, incandescence and luminescence.
Incandescence is light from heat energy. If you heat something to a high enough temperature, it will begin to glow.
When an electric stove’s heater or metal in a flame begin to glow “red hot”, that is incandescence. When the tungsten filament of an ordinary incandescent light bulb is heated still hotter, it glows brightly “white hot” by the same means.
The sun and stars glow by incandescence.
Luminescence is “cold light” that can be emitted at normal and lower temperatures.
In luminescence, some energy source kicks an electron of an atom out of its lowest energy “ground” state into a higher energy “excited” state; then the electron returns the energy in the form of light so it can fall back to its “ground” state. With few exceptions, the excitation energy is always greater than the energy (wavelength, color) of the emitted light.
If you lift a rock, your muscles are supplying energy to raise the rock to a higher-energy position. If you then drop the rock, the energy you supplied is released, some of it in the form of sound, as it drops back to its original low-energy position.
It is somewhat the same with luminescence, with electrical attraction replacing gravity, the atomic nucleus replacing the earth, an electron replacing the rock, and light replacing the sound.
Become a Member
As a member driven 501(c)3 scientific and education based charitable organization, we rely on public support for our efforts. Currently, we have nearly 400 members from all over the globe and greatly appreciate their support. Come and join the fun!
Common Varieties of Luminescence in Minerals
Fluorescence and Photoluminescence
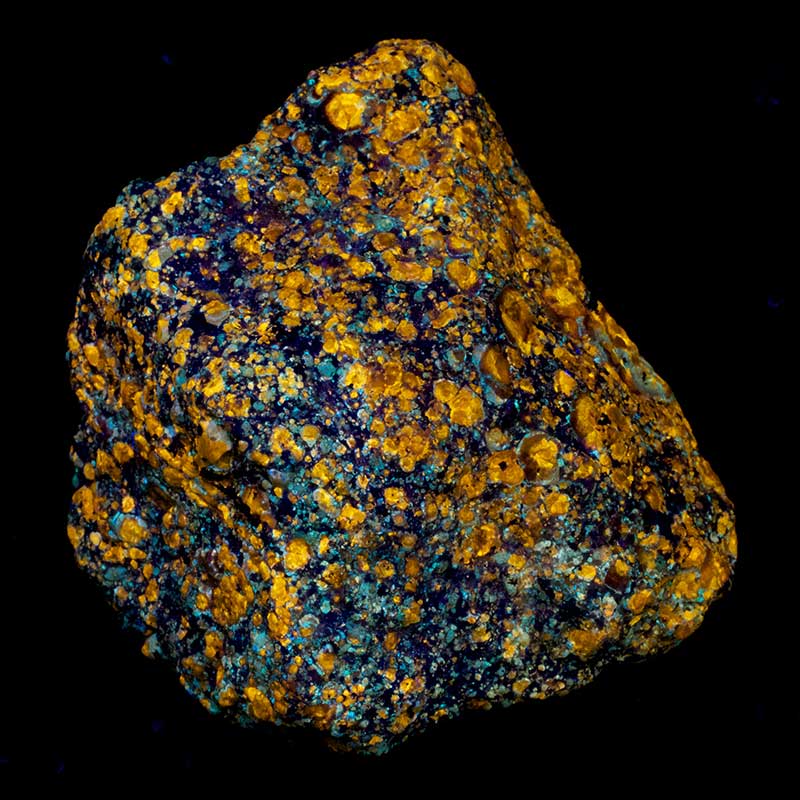
Fluorescence and Photoluminescence are luminescence where the energy is supplied by electromagnetic radiation (rays such as light, discussed in the section on UV). Photoluminescence is generally taken to mean “luminesce from any electromagnetic radiation”, while fluorescence is often used only for luminescence caused by ultraviolet, although it may also be used for other photoluminescences.
Fluorescence is seen in fluorescent lights, amusement park and movie special effects, the redness of rubies in sunlight, “day-glo” or “neon” colors, and in emission nebulae seen with telescopes in the night sky. Bleaches enhance their whitening power with a white fluorescent material.
Photoluminescence should not be confused with reflection, refraction, or scattering of light, which cause most of the colors you see in daylight or bright artificial lighting. Photoluminescence is distinguished in that the light is absorbed for a significant time, and generally produces light of a frequency that is lower than, but otherwise independent of, the frequency of the absorbed light.
Phosphorescence
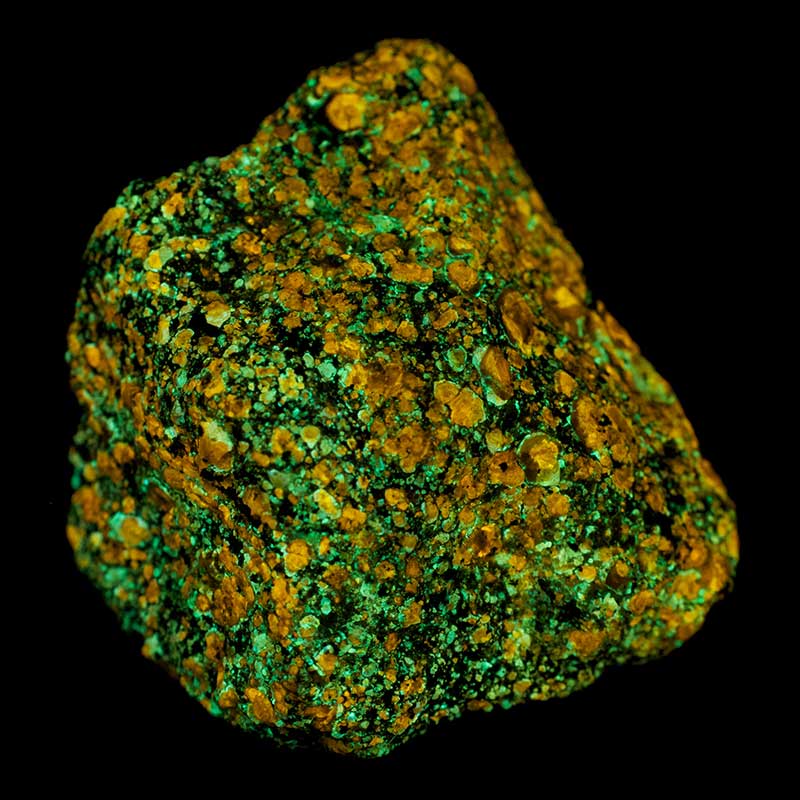
Phosphorescence is delayed luminescence or “afterglow”. When an electron is kicked into a high-energy state, it may get trapped there for some time (as if you lifted that rock, then set it on a table).
In some cases, the electrons escape the trap in time; in other cases they remain trapped until some trigger gets them unstuck (like the rock will remain on the table until something bumps it). Many glow-in-the-dark products, especially toys for children, involve substances that receive energy from light, and emit the energy again as light later.
Triboluminescence
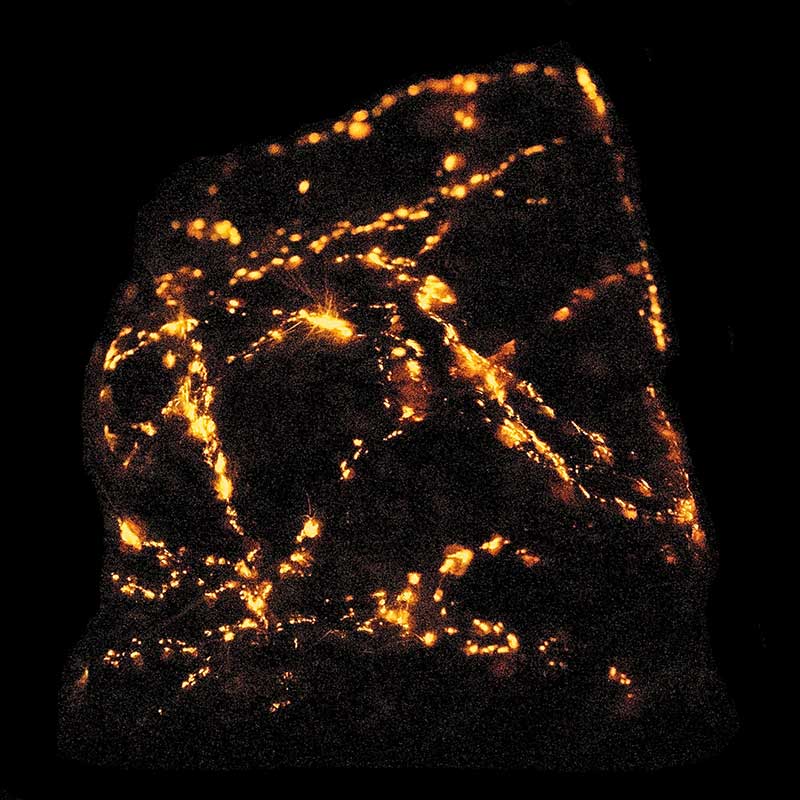
Triboluminescence is phosphorescence that is triggered by mechanical action or electroluminescence excited by electricity generated by mechanical action. Some minerals glow when hit or scratched, as you can see by banging two quartz pebbles together in the dark.
(The visible light emitted is often a secondary fluorescence effect, from electroluminescence in the ultraviolet).
Thermoluminescence

Thermoluminescence is phosphorescence triggered by temperatures above a certain threshold. This should not be confused with incandescence, which occurs at higher temperatures.
In thermoluminescence, heat is not the primary source of the energy, only the trigger for the release of energy that originally came from another source. It may be that all phosphorescences have a minimum temperature, but many have a minimum triggering temperature below normal temperatures and are not normally thought of as thermoluminescences.
Other varieties of luminescence
Chemiluminescence is luminescence where the energy is supplied by chemical reactions. Those glow-in-the-dark plastic tubes sold in amusement parks are examples of chemiluminescence.
Bioluminescence is luminescence caused by chemical reactions in living things; it is a form of chemiluminescence. Fireflies glow by bioluminescence.
Electroluminescence is luminescence caused by electric current.
Cathodoluminescence is electroluminescence caused by electron beams; this is how television pictures are formed on a CRT (Cathode Ray Tube). Other examples of electroluminescence are neon lights, the auroras, and lightning flashes.
This should not be mistaken for what occurs with the ordinary incandescent electric lights, in which the electricity is used to produce heat, and it is the heat that in turn produces light.
Radioluminescence is luminescence caused by nuclear radiation. Older glow-in-the-dark clock dials often used a paint with a radioactive material (typically a radium compound) and a radioluminescent material.
The term may be used to refer to luminescence caused by X-rays, also called photoluminescence.
Optically stimulated luminescence is phosphorescence triggered by visible light or infrared. In this case red or infrared light is only a trigger for release of previously stored energy.